Pfizer officially requested FDA approval for their vaccine in children, low-dose of the vaccine to be approved for children by Thanksgiving
It looks like we are getting closer to the period when the Pfizer vaccine will be allowed for children in the United States.
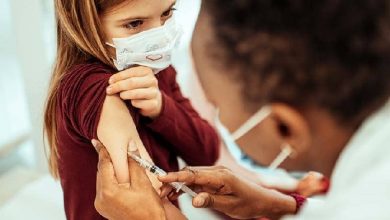
Few days ago, Pfizer officially sent the emergency use approval request to the FDA requesting authorization of their low-dose vaccine in children aged 5-11.
Taking into consideration to previous approvals, the company and some health experts believe that if everything goes as planned, the authorization of the vaccine will be given no later than Thanksgiving.
The pediatric COVID-19 vaccine is a low-dose version of its regular shot, containing just one-third of the amount given to adults and teenagers. In tests, it offered similarly strong protection against the virus.
“You want to maximize protection with the fewest amount of side effects,” Dr. Grace Lee, a pediatrician, said.
Although majority of children can tolerate the virus if they get infected with Covid-19, there are some that end up in hospital with severe condition and this was especially notable with the latest Delta wave.
“Sometimes we’re not able to predict well who will suffer severe consequences,” Lee said.
The Food and Drug Administration’s Vaccine Advisory Committee will discuss Pfizer’s request on Oct. 26.
Pfizer is also developing vaccines for babies and toddlers under the age of 5. Those vaccines are not expected to be ready before next year.



Leave a Reply
You must be logged in to post a comment.